Parasites (Pteromalus venustus) of
Alfalfa Leafcutting Bees
Pteromalus venustus^ is a very small wasp that parasitizes alfalfa leafcutting bees (ALB) (Megachile rotundata) and can cause serious economic loss. They are sometimes referred to as parasites, Pteromalus, chalcid wasps, chalcidoids, or parasitoids. These terms are not necessarily specific to P. venustus, so context is important. In this summary, P. venustus will be referred to most often as “parasites” which is commonly used by ALB producers.
Parasites are a serious problem for a number of reasons. These include the high number of offspring (brood) per parasitized bee cocoon, short generation period, limited control options, and limited acceptance in the market place. Parasitism of over 50% of bee cocoons has been recorded in western Canada12, but even levels around 1% or less can be economical depending on markets. |
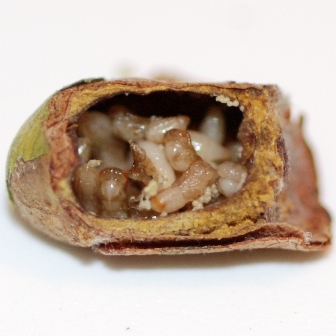
Adult female P. venustus |
On ALB cocoon |
Life Cycle of Parasites in the Incubator
This is a general description of parasite development and activity during the incubation period. Understanding the lifecycle can help to better manage this damaging and impressive insect. The stage of development of the bee or parasite can depend on a number of factors such as temperature and uniformity of incubation. The schedule presented here assumes incubation at about 30oC.
Pteromalus venustus overwinters as a prepupa8 (mature larva2) and it may be seen at this stage, in a closed bee cocoon (a.k.a. cell), at the start of incubation (Figs. 1-3). Parasites that are stored all winter as eggs or adults do not survive8, and so the number of cocoons with viable parasites may be reduced over winter, while adults stored at 5oC for 33 days can survive but do not lay eggs when warmed up8. Parasite larvae held at 5oC for more than a year have been found to show normal development when incubated at room temperature8.
At the start of incubation, there is no viable bee in a parasitized cocoon because the developing parasites consume most of it12. Parasite larvae may also consume each other8,12. There may be an average of about 32 parasite larvae in a single bee cell8 at the start of bee incubation, and an average of about 17 parasite adults have been found to emerge from individual bee cocoons12. The number of male and female parasites in a bee cocoon is about equal (i.e. 1:1 ratio)8,12.
In the incubator, parasites develop from prepupa to adult relatively quickly (Figs. 2-8), and new adults usually begin to emerge from cocoons around day 8-126 or possibly earlier. Parasite larval and pupal development increases with temperature from 20 to 32oC, but there is no larval development at 10 or 15oC12. To get out of the closed bee cocoon, they chew a single hole in it (Fig. 9), and the adults emerge one at a time (Figs. 10 & 11). Adult parasites may be seen in the trays and on surfaces in the incubator (Fig. 12). Adult parasites can live for about two weeks without a feed (honey syrup)8.
Mating of parasites can occur “within seconds” of females shedding their pupal skins8. Mating may be seen outside the bee cocoon (Fig. 13) and involves some precopulatory routine8. Females usually mate once only8.
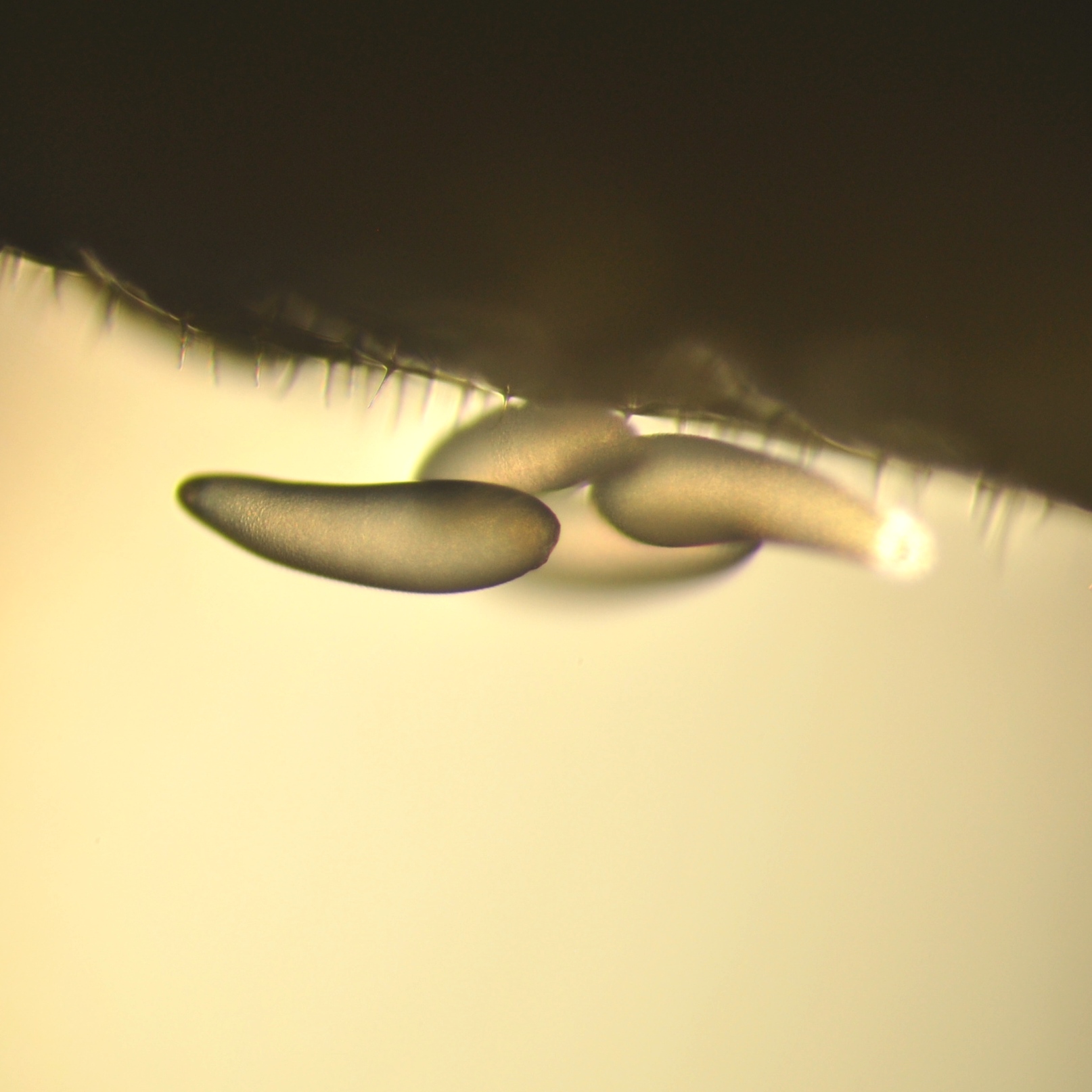
Parasitism in the incubator occurs at this critical juncture - around day 8-12, in particular - when parasites mature and emerge, females are mated, and the bees are at the susceptible prepupal stage. The adult female parasite inserts her ovipositor through the bee cocoon (Fig. 14) and into the immature bee to anesthetize it, and then she lays her eggs on the surface of the bee8. Twenty-five percent (25%) of parasitism can happen within 4 hours of parasite emergence from the bee cocoon (Richards Unpublished Data6). The parasites consume the bee almost entirely before they become pupae12.
Each newly parasitized cocoon represents a killed bee and a host for many more new parasites. Since a parasite can complete a generation in the incubator in about 12 days12, a second generation of adult parasites may be visible around day 21 (Fig. 12). At this point, the surviving bees are at or near adult stage and are not susceptible to parasitism, but this second generation of parasites may go on to parasitize cocoons in the field, and continue the problem.
Parasites exhibit a facultative diapause8 of suspended development in response to environmental conditions, or diapause may also occur when parasites are at a constant temperature (Goerzen pers. comm.). Their diapause can be ended when they are warmed up after storage at 5oC for about 2 months8. The ALB on the other hand exhibits a diapause at the prepupal stage, more often than not during each generation, which is ended after winter storage when the bees are incubated. The triggers and mechanisms for the development of “second generation” ALBs are not known for certain10.
|
Fig 1. ALB cocoon |
Fig 2. Parasite larvae in bee cocoon (cut open to show inside) |

Fig 3. Parasite larva |
Fig 4. Immature parasites at various stages of development in bee cocoon
(cut open to show inside) |
Fig 5. Parasite pupa |
|
|
Fig 7. Parasite pupa |
Fig 8. Parasite adult female,
recently emerged |
Fig 9. ALB cocoon with
parasite emergence hole |
|
Fig 10. Parasite adult emerging
from ALB cocoon |
Fig 11. Parasite adults on
ALB cocoon |
Fig 12. Parasite adults,
male and female |
|
Fig 13. Parasite adults mating |
Fig 14. Parasitism of
prepupal bee in cocoon
(cut open to show inside) |
Fig 15. Parasite eggs on
surface of an ALB larva |
Management of Parasites (Research Summary)
Because parasites can cause serious economic loss, when buying bees for the first time, bee cocoons should be purchased that are free of parasites. This can be determined by having representative cocoons analysed, although parasites may still present at relatively low levels even when undetected in samples.
Even with cocoons that enter incubation with relatively few parasites, levels can get very high quickly if not managed. If a problem with parasites is new or isolated, it may be advantageous to incubate or manage the cocoons separately to try to contain it. This would likely involve extensive and on-going sampling to determine the size and extent of the issue.
Dichlorvos Treatment
(***Note regarding Dichlorvos, The Ortho Home Defense Max No Pest Insecticide Strip product (Reg. No. 22027) has been cancelled and has a last date of use of August 20, 2023. Users are no longer permitted to use this product in Canada after August 20, 2023.
Light And Water Trap
A light and water trap is often used to help manage parasites7. A black light (ex: black light fluorescent tube) is set up over a pan or tub with water, and a small amount of surfactant (ex: a few drops of liquid soap) is put in the water to reduce the surface tension so the parasites drown more effectively. Adult parasites are attracted to the ultraviolet light emitted from black light tubes/bulbs and drown in the water. This trap can be set up for the duration of the incubation period and in the storage room for nests with cocoons in the fall as well. The water may need to be cleaned of insects periodically.
Nest Assembly
Good nest construction is needed to protect bee cocoons from parasites. There are a number of nest types and surrounds but the principles are the same. The tunnel openings at the back of the nest must be protected with bonded polyester material and a backing board secured tightly to the block. Pushing the nest down during strapping may help make it tight. The eggs at the back of the block are laid first, reach the prepupal stage earlier and therefore are susceptible to parasitism the soonest. Significantly more brood has been found to be parasitized at the back of the laminate polystyrene nest tunnel, in positions 1-3, which contain mostly female bees, compared to cells nearer the entrance of the nest tunnel, in positions 8-10, which contain mostly male bees14; however, which bees are parasitized may be influenced by general nest integrity. When the nest tunnel is full, the female constructs a nest plug made of leaf pieces to prevent parasites and other threats to her brood. Holes in the nest created by other insects or animals can also be used by parasites to reach cocoons. Therefore nests must be inspected regularly and culled or secured as needed. Any exposed cocoons with mature larvae may be parasitized. If the adult parasite can’t get at the prepupal bee, it can’t parasitize it.
Pyrethrin Aerosol
Pyrethrins are a class of organic compounds with insecticidal activity. Research has found that the use of pyrethrin aerosol in the incubator, when parasites are emerging or recently emerged from cells, can reduce the percentage of cocoons that are parasitized3. Even distribution of the pyrethrin particles in the incubator is important to kill parasites. Cans of pyrethrin aerosol have been used manually or in timed-release dispensers secured to the incubator wall facing the cocoon trays. Pyrethrin aerosol can also be a risk to the bees and therefore precautions should be taken to reduce the risk and prevent bee loss. Pyrethrins break down relatively quickly in the environment, especially when exposed to ultraviolet light or natural sunlight. Synergists in a product formulation may help prevent break down of the product1.
Mechanical Cocoon Sorting - A portion of the number of cells with parasites have been found to be removed in the process of sorting by weight, but this is not considered a primary management option. Humidity, and other factors, may affect how well cocoons can be sorted since they can absorb moisture from the air which adds weight.
In a study designed to help understand the impact of replacing partially-filled nests on the production of female bees, it was found that parasite levels were significantly lower (60-97% reduction) in the replacement nest blocks14. The possible reasons for this reduction in parasitism are speculated to include: fewer wasps later in the season, seasonal phenology or age-related mortality, or parasites are relatively poor flyers.
Parasites are a serious concern for ALB producers. Understanding their life cycle is important to managing them, and they require effective management to prevent serious economic loss. At the same time, the bees must be protected and kept safe at the critical incubation period and other times of the management year. For more information, contact Manitoba Agriculture.
End Notes:
^ Synonymized under Pteromalus apum (Retzius 1783) by Boucek and Graham (1978).
References:
1 ATSDR, Agency for Toxic Substances and Disease Registry. 2003. Public health statement for pyrethrins and pyrethroids.
2 Eves, J.D., D.F. Mayer and C.A. Johansen. 1980. Parasites, predators, and nest destroyers of the alfalfa leafcutting bee, Megachile rotundata. Washington State University WREP 32.
3 Goerzen, D.W. 2001. Alfalfa leafcutting bee parasite control with pyrethrin aerosols. Saskatchewan Alfalfa Seed Producers Association Extension Publication 2001-03.
4 Goerzen, D.W. 2011. Parasite control in alfalfa leafcutter bee populations – 2011. Saskatchewan Alfalfa Seed Producers Association.
5 Goerzen, D.W. and D.C. Murrell. 1992. Effect of fall dichlorvos treatment for control of a chalcid parasitoid on viability of the alfalfa leafcutting bee Megachile rotundata (Fabr.) (Hymenoptera: Megachilidae). Bee Sci. 2: 37-42.
6 Hill, B.D., K.W. Richards and G.B. Schaalje. 1984. Use of dichlorvos resin strips to reduce parasitism of alfalfa leafcutter bee cocoons during incubation. J. Econ. Entomol. 77: 1307-1312.
7 Hobbs, G.A. 1973. Alfalfa leafcutter bees for pollinating alfalfa in western Canada. Canada Department of Agriculture. Publication 1495.
8 Hobbs, G.A. and M.D. Krunic. 1971. Comparative behaviour of three chalcidoid (Hymenoptera) parasites of the alfalfa leafcutter bee, Megachile rotundata, in the laboratory. Can. Entomol. 103: 674-685.
9 Lehnert, M.P., R.M. Pereira, P.G. Koehler, W. Walker and M.S. Lehnert. 2011. Control of Cimex lectularius using heat combined with dichlorvos resin strips. Med. Vet. Entomol. 25: 460-464.
10 Pitts-Singer, T.L. and J.H. Cane. 2011. The alfalfa leafcutting bee, Megachile rotundata: the world's most intensively managed solitary bee. Annu Rev Entomol. 56: 221-237.
11 Purdy, J.R. and P.G. Kevan. 2014. Bioassay for detection of dichlorvos insecticide in air in alfalfa leafcutting bee (Megachile Rotundata F.) incubators. J. Apic. Sci. 58: 41-47.
12 Whitfield, G.H. and K.W. Richards. 1985. Influence of temperature on survival and rate of development of Pteromalus venustus (Hymenoptera: Pteromalidae), a parasite of the alfalfa leafcutter bee (Hymenoptera: Megachilidae). Can. Entomol. 117: 811-818.
13 U.S. Department of Health and Human Services. 1997. Toxicological Profile for Dichlorvos.
14 Jay, S.C. and N. Mohr. 1987. The effect of nest replacement on the production of females of the alfalfa leaf-cutter bee Megachile rotundata (F.). J. Apic. Res. 26: 69-72.